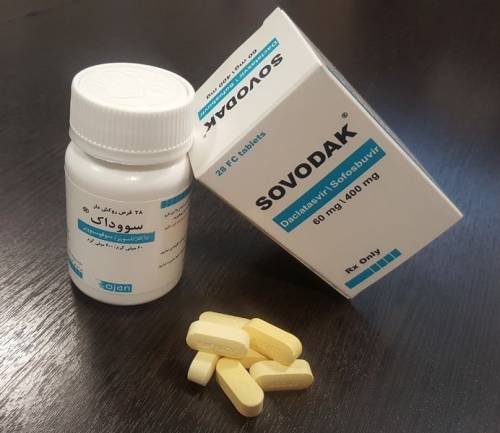
- Daclatasvir and Sofosbuvir, when used in combination, have proven to be highly effective in the treatment of Hepatitis C virus infection.
- This all-oral combination therapy is highly desirable for patients with chronic HCV infection, and is preferred over the traditional treatment methods which involved the use of interferons.
- Daclatasvir and Sofosbuvir are an important breakthrough in the treatment of Hepatitis C and provide hope for those who suffer from this debilitating disease.
What is Hepatitis C
Hepatitis C is a viral infection that, if left untreated, can have devastating consequences for a person’s health. Inflammation, liver injury, cirrhosis, and even liver cancer are all potential consequences. Blood transfusions and the use of contaminated syringes are the most prevalent means of transmitting HIV. In order to prevent irreversible liver injury, early diagnosis and treatment are crucial, as the majority of infected patients exhibit no symptoms.
Hepatitis C has catastrophic consequences if left untreated, including liver failure and mortality. This illness should be treated seriously; therefore, immediate medical attention should be sought. The good news is that in many cases, the infection is curable if treatment is initiated promptly. Therefore, if you have any reason to suspect that you may have been exposed to hepatitis C, you must undertake testing and treatment immediately.
There may be practical and financial barriers for some individuals to access testing and treatment, especially in low-income or under-resourced areas, which could hinder their ability to seek immediate medical attention.
Daclatasvir/sofosbuvir combined effect
If you or a loved one has been diagnosed with hepatitis C, there is hope for effective treatment with Daclatasvir and Sofosbuvir. Here’s how to get started:
- Step 1: Speak to a medical professional
Before beginning any treatment, it’s important to speak with a medical professional who can diagnose and evaluate your condition. They will be able to recommend the best treatment plan for you based on your individual needs and medical history. - Step 2: Consider a combination therapy
Recent studies have shown that combination therapy with Daclatasvir and Sofosbuvir is highly effective in treating hepatitis C. This treatment works by targeting specific proteins in the hepatitis C virus, preventing it from replicating and allowing the body to eliminate the virus. - Step 3: Follow the prescribed dosage
The recommended dosage for Daclatasvir and Sofosbuvir varies based on the patient’s condition and genotype. It’s important to follow the prescribed dosage and duration of treatment to ensure its effectiveness. - Step 4: Monitor side effects
While Daclatasvir and Sofosbuvir have been shown to be generally well-tolerated, patients should be aware of potential side effects like headaches, fatigue, and nausea. If any side effects are experienced, patients should speak to their medical professionals who can offer recommendations for managing them.
So, treating hepatitis C with Daclatasvir and Sofosbuvir is an effective option to eliminate the virus and improve the overall health of patients. Remember to speak to a medical professional, consider combination therapy, follow the prescribed dosage, and monitor side effects for the best results. With proper treatment and care, hepatitis C can be treated and managed successfully.
How long is the treatment for Sofosbuvir and Daclatasvir?
If you or someone you know has been diagnosed with hepatitis C, you may be wondering how long the treatment for Sofosbuvir and Daclatasvir lasts.
According to factual data, the recommended duration of treatment varies depending on the specific genotype of the virus and the severity of liver disease. In general, treatment lasts for 12-24 weeks, with some patients requiring additional medication like ribavirin.
It’s important to discuss your specific situation with a healthcare provider, who will be able to recommend the appropriate treatment and duration based on your individual needs.
Even though the length of treatment may seem long, it’s important to finish all of your medications to give yourself the best chance of getting better and having a healthier future. Hepatitis C can be treated and even cured with the right help and care.
What is the success rate of these medications?
Two direct-acting antiviral medications used to treat hepatitis C virus (HCV) infection are daclatasvir and sofosbuvir. Clinical studies using these medications to treat HCV have had very encouraging results.
The figures demonstrate the extremely high success rate of daclatasvir. Daclatasvir with sofosbuvir had a ~97.5% overall sustained virological response (SVR) rate in cirrhotic individuals. Another drug with a high success rate is sofosbuvir. In the mITT group, ~93.9% of patients with HCV recurrence after liver transplantation attained SVR12.
In a variety of clinical studies, both daclatasvir and sofosbuvir showed very high levels of both effectiveness and tolerability. In chronic HCV patients, the SVR12 rates at 12 weeks were similar between both of these medications.
Daclatasvir is an effective inhibitor of the HCV NS5A replication complex, while sofosbuvir is a nucleotide analog that inhibits the HCV RNA polymerase.
By offering excellent cure rates to patients with a range of HCV genotypes, we are changing the HCV therapy landscape. In conclusion, daclatasvir and sofosbuvir are extremely successful and tolerated for the treatment of HCV infection, according to the data. Patients with varied HCV genotypes, including cirrhotic patients and those with post-liver transplant HCV recurrence, had extremely good success rates with them.
About the Author
Reyus Mammadli is the author of this health blog since 2008. With a background in medical and biotechnical devices, he has over 15 years of experience working with medical literature and expert guidelines from WHO, CDC, Mayo Clinic, and others. His goal is to present clear, accurate health information for everyday readers — not as a substitute for medical advice.