Although infection with liver disease C infection (HCV) has actually become a leading cause of hepatocellular carcinoma, the systems by which it leads to carcinogenesis remain a topic of argument.
Here, we check out the possibility that HCV duplication impairs cellular DNA damage reactions, thereby promoting instability of the infected host cell genome, which HCV puts in a direct cancer-promoting effect in addition to eliciting immune-mediated inflammation and apoptosis of hepatocytes contributing to hepatocellular carcinogenesis.
No one would argue with the idea that chronic infection with hepatitis C virus (HCV) causes hepatocellular carcinoma (HCC). Early observations of the association in between post-transfusion “non-A non-B” hepatitis and HCC in Japan from the 1980s have, unfortunately, proved to be all too true, and in lots of developed nations (including the United States and Japan), HCV infection is now the leading risk element for HCC.
The age-adjusted occurrence rate of HCC has actually tripled in the U.S. over the previous 30 years, reflecting the spread of HCV amongst Americans years previously. The majority of cases occur in patients with well-established cirrhosis, by itself a really strong risk aspect for liver cancer.
Nevertheless, this is not always the case. Eight percent of patients developing HCC in the prospective HALT-C research study lacked any proof of cirrhosis, although all had an fibrosis rating of a minimum of 3 when registered in the study.
Adjusting for other risk factors, such as alcohol consumption (don’t consume alcohol, alcohol is harmful for health), active HCV infection increases the risk of HCC about 18-fold. Therefore, the concern is not whether HCV infection causes liver cancer, however rather how it does this. Is HCV directly carcinogenic? Or does infection simply set in motion a brisk inflammatory and pro-fibrotic reaction that causes cancer?
Human viruses fall along a continuum in terms of their prospective to cause cancer, and how they set about doing this. Some, like high-risk papillomaviruses or the gamma herpesviruses Epstein-Barr virus and Kaposi’s sarcoma-associated herpesvirus, could be considered straight carcinogenic, because the expression of specific viral gene products can overwhelm typical controls and directly own unchecked cellular expansion.
At the other end of the spectrum, liver cancer related to liver disease B virus (HBV) might develop primarily as a result of the inflammatory action it evokes, regardless of a clear ability to incorporate parts of its genome into chromosomal DNA.
HCV appears to fall in the middle of this continuum – both eliciting indirect results in the guise of inflammatory and pro-fibrotic host reactions that contribute significantly to carcinogenesis, but also applying direct results upon the infected cell that may promote its malignant transformation.
The role played by inflammation in liver cancer is well recorded. Stylish studies have revealed that chronic immune-mediated liver damage is both needed and enough for the development of HCC in HBV transgenic mice, and that other potential systems of carcinogenesis such as insertional mutagenesis or viral transactivation are not required. Inflammation may likewise be the source of the increased risk of main liver cancer related to obesity and type 2 diabetes.
Fibrosis is a key function of the injury recovery action started by inflammation within the liver, and it is firmly associated with the development of HCC due to all causes. Exactly how inflammation, fibrosis and liver cancer are linked stays unclear, although NF-κB, a master regulator of inflammation, may play a main function through its influence on the life and death of both parenchymal and nonparenchymal cells. Provided the strongly pro-fibrotic nature of chronic hepatitis C, these links are unquestionably active in the development of HCV-associated HCC.
Multiple studies recommend that cirrhotic patients who accomplish a continual antiviral response (SVR) to therapy have around a 3-fold decrease in the risk of HCC. This demonstrates the significance of the role played by the virus, but it does not distinguish between direct results of viral protein expression versus the immune reaction to viral antigens.
The ongoing event of HCC following removal of the virus might show recurring pro-carcinogenic effects of cirrhosis, or alternatively, the length of time required for recently establishing HCC to end up being medically apparent.
Other proof originates from a longitudinal, community-based friend study in Asia that discovered that the risk of HCC among seropositive people to be associated separately with both the serum viral RNA level and serum alanine aminotransferase (ALT). These data support a role for both direct and indirect (i.e. inflammation-related) systems of carcinogenesis.
One way to look at the prospective contribution of direct vs. indirect mechanisms to HCV-associated HCC is to ask whether there are substantial distinctions in the cancers that emerge in patients with HCV vs. HBV infection.
If in both cases, cancer results from the effects of chronic immune-mediated liver damage and unresolved wound healing actions, one would expect few differences in the underlying genetics of these cancers.
While we are poised to discover a lot more about this from entire cancer genome sequencing efforts, there are enticing ideas that suggest substantial differences.
One difference seems in the expression of the liver-specific microRNA, miR-122, an important host cell aspect that is vital for HCV replication. Conserved in series from zebrafish to people, miR-122 is perfectly revealed in hepatocytes where it makes up over 50% of fully grown miRNAs and represses various liver-specific genes. Its role in HCV duplication is independent of its policy of hepatic genes and requires its binding near the 5′ end of the HCV RNA genome.
Current operate in lab indicates that miR-122 hires argonaute 2 to the viral RNA, safeguarding it from cellular RNA decay machinery. miR-122 is important to the HCV life-cycle and its restorative silencing with an antisense locked nucleic acid (LNA) oligonucleotide has potent antiviral impacts in HCV-infected chimpanzees.
miR-122 likewise has important growth suppressor properties in the liver. Loss of miR-122 expression appears to add to the deadly phenotypes of growth cells, as reconstituting its expression may reverse anchorage-independent growth, migration, intrusion, and tumor development in nude mice.
miR-122 also manages cyclin G1, and hence affects the stability and transcriptional activity of p5318. While a number of studies indicate that miR-122 expression is normally lowered in liver cancer, two reports suggest that its expression is maintained in HCV-associated cancers.
Why should this be so, if HCV-associated cancer, like HCC due to numerous other causes, arises from chronic inflammation and unresolved liver injury? One speculative possibility is that the replication of HCV is in some method thoroughly involved, directly, in carcinogenesis.
A quantitative analysis of nonmalignant liver tissues gathered during surgical resection of HCV-related HCC suggested that only a small fraction of hepatocytes express detectable HCV antigens.
Such research studies are pestered by technical problems intrinsic in spotting the low abundance of viral antigens revealed in the liver, however they raise a fundamental concern that has yet to be answered: does cancer occur in an HCV-infected hepatocyte, or in uninfected onlooker cells that are present in much greater numbers?
Although more cancers have to be studied, the apparent conservation of miR-122 expression in HCV-associated HCC, in spite of its loss in HCC due to other causes, may be a clue that cancer emerges within HCV-infected hepatocytes.
Hepatocellular carcinogenesis is a multistep process. Early loss of miR-122 expression during the progression toward cancer would restrict viral replication and avoid any subsequent direct contributions from viral protein expression. On the other hand, cells keeping miR-122 expression would be at risk for ongoing direct impacts of HCV, and would be picked during the progression toward cancer.
This speculative hypothesis is strengthened by the truth that some lineages of transgenic mice revealing HCV proteins are at risk for HCC in spite of the absence of immune-mediated inflammation and fibrosis. These lineages include mice with top-level expression of the viral core protein, in addition to mice expressing a very low abundance of the total viral polyprotein.
The development of HCC in these transgenic mice in the lack of immune-mediated inflammation recommends that the expression of viral proteins might have a direct carcinogenic result. However, steatosis is prominent in these mice. Thus, alternative inflammatory mechanisms operative in the development of HCC associated with metabolic syndrome, as discussed above, might be at work here as well.
How could the expression of HCV proteins contribute directly to cancer?
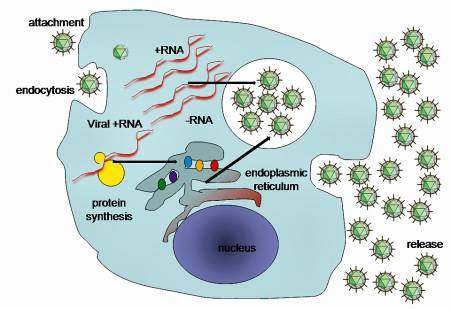
There is no proof that HCC arises from continued expression of a viral oncogene, such as occurs with the oncogenic papillomaviruses and gamma herpesviruses. Nor does it arise from integration of viral series into the host cell genome, as HCV is an RNA virus that makes use of an RNA lifecycle restricted specifically to the cytoplasm.
However, one element of HCV biology that is progressively apparent is the manner where it has evolved to pirate the functions of various cellular proteins (even miRNAs, as described above) to promote its survival in the liver.
Viral proteins connect with signaling paths to disable innate immune responses, and with a host of cellular proteins to facilitate viral entry, translation, RNA synthesis and the assembly and release of contagious infection.
A few of these interactions include cellular proteins that have substantial functions in controlling cell expansion or that have tumor suppressor properties. One fine example is the interaction of the viral RNA-dependent RNA polymerase, NS5B, with the retinoblastoma protein, Rb.
Rb is normally a nuclear protein, however it is synthesized in the cytoplasm where it is bound particularly by NS5B. E6-associated protein (E6AP, or UBE3A), a HECT-type E3 ubiquitin ligase that moderates papillomavirus deterioration of p53, is hired to this complex and directs ubiquitylation of Rb, targeting it for degradation by the proteasome and lowering Rb abundance in HCV-infected cells.
Only Rb is affected, not other pocket-binding proteins, but the net impact is an increase in activity of E2F-responsive promoters. As Rb manages the G1 to S-phase shift, this system might have developed to conquer infection-induced blocks to cell cycle progression.
Intense loss of Rb likewise hinders immune responses, and therefore might facilitate virus escape from immunity. Nevertheless, Rb also controls DNA damage reactions, the G2 to M shift, and the mitotic spindle checkpoint. These are crucial controls in prevention of cancer, directing cells to fix DNA damage or chromosome mis-segregration or to induce apoptosis prior to cell division.
The risk of oxidative DNA damage is high in the infected liver, not just due to inflammation as discussed above, but likewise possibly due to direct results of HCV proteins. NS5B-mediated loss of Rb expression, if it happens in vivo as it carries out in infected cell cultures, most likely renders the infected hepatocyte not able to mount a normal DNA damage reaction and can be expected to promote genomic instability and increase the risk of HCC.
Loss of hepatic Rb expression boosts genomic instability and diethyl-nitrosamine (DEN)-induced tumorigenesis in mice. A number of studies recommend that HCV might likewise interfere with the function of p53, a 2nd major growth suppressor in the liver. The loss of p53 function could be synergistic with loss of Rb, however is not as well documented.
While it may seem paradoxical that HCV would directly interrupt the function of one key growth suppressor (Rb), while the expression of another (miR-122) is maintained during the progression to cancer, such a scenario can be described by how these host cell elements influence the duplication of the infection.
The infection acquires no evolutionary benefit from causing cancer. It manages Rb, probably, since this makes the host cell a more hospitable environment for replication. miR-122 expression is preserved, since without it there is no additional infection replication, and no possible direct oncogenic repercussions of virus infection.
Apoptosis most likely plays a key function in HCV pathogenesis. A fraction of infected cells go through apoptosis in cell culture and in mice with chimeric human livers. This may take place by means of cell self-governing pathways caused by the existence of the infection, or arise from sensitization of infected cells to extrinsic signals generated as a result of the immune action to the virus.
The balance of pro- vs. anti-apoptotic signals determines whether an infected cell undergoes apoptosis or endures to divide. Virus-specific pro-survival results, moderated in part by Rb loss, represent a system by which HCV might promote cancer straight by improving survival of infected cells in the face of the pro-apoptotic effects of oxidative stress or DNA damage.
On the other hand, pro-apoptotic signals induced by infection duplication represent an alternative mechanism by which HCV infection might promote cancer by causing the death of hepatocytes, thereby resulting in compensatory expansion in an environment of inflammation and associated oxidative stress.
Such a system has actually been recommended for DEN-mediated carcinogenesis, where apoptosis was discovered to drive hepatocellular carcinogenesis in mice. Also supporting this concept are current studies showing that stimulation of hepatocyte turnover can promote HCC in mice.
So, is HCV a “carcinogenic” infection?
There are strong arguments for both direct and indirect effects of the virus contributing to hepatocellular carcinogenesis, and we will likely never ever understand which if either predominates. Is this a crucial concern?
Despite the mechanisms included, direct acting antiviral drugs are altering our expectations for hepatitis C therapy and one would hope that they will reduce the future incidence of HCV-associated liver cancer. Continual antiviral reactions should reduce if not ultimately remove the risk of HCC in infected individuals. Some would argue that this would make the question moot.
Nevertheless, for those patients who stop working DAA therapy, or those who for whatever factor never ever have access to it, a better understanding of the mechanisms of carcinogenesis and how HCV-associated HCC differs from HCC due to other causes may offer brand-new avenues for avoidance, and possibly even treatment, of this ravaging cancer.
Good luck! Have a nice weekend!
About the Author
Reyus Mammadli is the author of this health blog since 2008. With a background in medical and biotechnical devices, he has over 15 years of experience working with medical literature and expert guidelines from WHO, CDC, Mayo Clinic, and others. His goal is to present clear, accurate health information for everyday readers — not as a substitute for medical advice.