Aspergillus fumigatus is one of the most common types of mold found almost everywhere — in soil, decaying leaves, household dust, and even the air we breathe. For those wondering what is Aspergillus fumigatus, it’s a saprophytic fungus that feeds on decomposing organic matter. Under normal conditions, it lives quietly in the background of nature’s recycling system, invisible yet ever-present.
However, is Aspergillus fumigatus dangerous? For most healthy individuals, it’s harmless. But when a person’s immune system is weakened, this same mold can turn aggressive, triggering respiratory infections and severe allergic symptoms. That’s when a silent environmental organism can suddenly become a serious medical threat.
Six-Week Mortality Rates for Invasive Aspergillosis by Country
This chart illustrates the six-week mortality rates for invasive aspergillosis across selected countries. The values reflect variations in healthcare outcomes, treatment efficacy, and diagnostic approaches among nations.
Why Is It a Rising Threat?
Here’s the kicker: Aspergillus fumigatus is evolving fast, and that’s not great news. Over the past decade, surveillance studies have documented a worrying rise in azole-resistant strains—azole drugs being frontline treatments for fungal infections. Recent global review data show environmental and clinical isolates of A. fumigatus with azole resistance rates sometimes exceeding 30 % in certain regions. ⧉
Factors contributing to this include:
- Pervasive use of azole fungicides in agriculture, which creates environmental hotspots for resistance development.
- Growing numbers of immunosuppressed patients (for example, individuals with cancer, transplant recipients, or those recovering from severe COVID-19) who are more vulnerable to opportunistic fungal pathogens.
- Climate change accelerating spore survival and dispersal—warmer compost-like conditions, higher ambient temperatures and humidity help A. fumigatus thrive in niches previously less favourable.
Who Is at Risk?
While healthy individuals usually breathe in A. fumigatus spores without consequence, the situation changes dramatically for those with:
- Chronic lung diseases (like COPD or asthma)
- Weakened immune systems (from chemotherapy, HIV/AIDS, organ transplants)
- Prolonged ICU stays
In the U.S., cases of invasive aspergillosis have been rising. For example, a 63-year-old male cancer patient from Houston developed an aggressive A. fumigatus lung infection that resisted standard treatments and required combination antifungal therapy.
Symptoms to Watch For
Depending on the form of aspergillosis, symptoms can vary in intensity, timing, and how they manifest during daily activities. It’s crucial to understand these subtleties to distinguish the infection from more common conditions like seasonal allergies or bacterial pneumonia.
Allergic bronchopulmonary aspergillosis (ABPA): Often affects individuals with asthma or cystic fibrosis. Wheezing becomes more persistent and less responsive to inhalers. Thick, brownish mucus plugs may appear during coughing, and shortness of breath worsens after exposure to dust, damp air, or compost-like environments. Nighttime coughing is especially common, and symptoms tend to be chronic, fluctuating with environmental triggers.
Chronic pulmonary aspergillosis (CPA): Fatigue gradually intensifies and doesn’t improve with rest. Unintentional weight loss persists even with a stable diet. The cough often produces sputum streaked with blood and worsens over weeks rather than days. A low-grade fever, often below 100.4 °F (38 °C), can linger for weeks, while breathlessness increases with mild exertion such as climbing stairs. Symptoms frequently resemble tuberculosis or lung cancer, delaying diagnosis.
Invasive aspergillosis: The most severe form, typically seen in immunocompromised patients. Fever is high and unresponsive to antibiotics. Chest pain may be sharp and pleuritic, worsening with deep breaths or coughing. Hemoptysis (coughing up blood) can start subtly and escalate rapidly. If left untreated, respiratory failure may develop, and in some cases the infection spreads to the brain, causing confusion or seizures. Early warning signs include rapidly worsening fatigue, oxygen desaturation with minimal effort, and a sense of overall decline.
Primary (Red Flag) Symptoms:
- Persistent high fever unresponsive to antibiotics
- Hemoptysis (even minor)
- Sharp chest pain with breathing or coughing
- Rapidly worsening shortness of breath
Secondary (Non-specific) Symptoms:
- General fatigue that lasts more than two weeks
- Mild fever below 100.4°F (38°C) with no obvious source
- Unexplained weight loss or appetite decline
- Night sweats or vague chest tightness
Understanding the timeline and progression of these symptoms is critical. A cough that lasts more than three weeks, even without fever, in a patient with known risk factors (e.g., chemotherapy, transplant, or prolonged ICU stay) should trigger a fungal investigation. Symptoms that come and go depending on environment or activity level should not be dismissed.
Recurrent or unexplained respiratory symptoms warrant early consultation with a pulmonologist or infectious disease specialist. The earlier aspergillosis is caught, the better the chances for effective treatment and recovery.
Diagnostics: Precision is Key
Diagnostic Accuracy of Tests for Invasive Aspergillosis
This chart visually compares the sensitivity (blue) and specificity (green) of diagnostic tests for invasive aspergillosis. PCR on BAL fluid shows the highest accuracy across both metrics, while other tests vary in diagnostic precision.
Early and accurate diagnosis of A. fumigatus infections is crucial.
| Diagnostic Test | Method | Accuracy (1-10) | Average Cost (USD) |
|---|---|---|---|
| Chest CT scan | Visual imaging of lung abnormalities | 8.5 | $500-$2,000 |
| Galactomannan antigen test | Detects fungal cell wall component | 8 | $90-$150 |
| PCR assay (fungal DNA) | Identifies fungal DNA in blood/tissue | 9.2 | $300-$600 |
| Bronchoalveolar lavage (BAL) | Fluid collection from lungs for culture | 9 | $1,000-$2,500 |
| Fungal culture and microscopy | Direct identification under a microscope | 7.5 | $200-$400 |
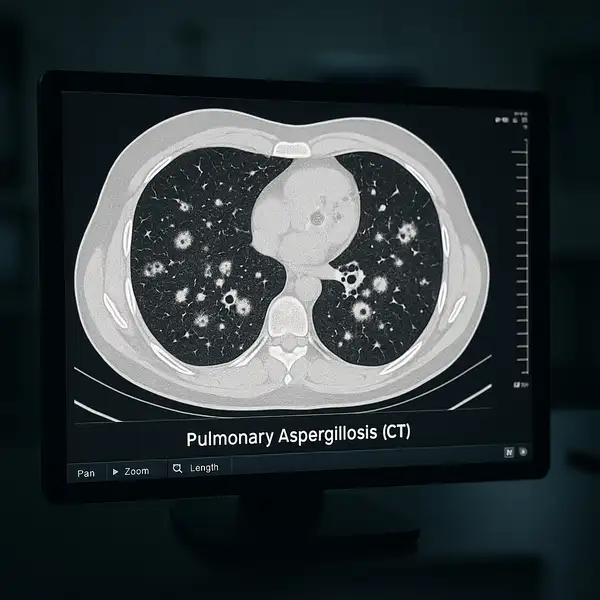
Update 2025: A new real-time PCR-based assay on the Panther Fusion system now allows simultaneous detection of Aspergillus fumigatus and Pneumocystis jirovecii with excellent sensitivity and specificity. In addition, researchers are employing Tip-Enhanced Raman Spectroscopy (TERS) for high-resolution chemical mapping of the fungal cell wall—an innovation enhancing understanding of pathogenic variability.
Treatment: Innovations and Challenges
Treatment depends on the severity and form of aspergillosis. Effective management often requires a personalized approach, and treatment availability, cost, and effectiveness can vary widely.
Efficacy of Antifungal Agents Against Aspergillus fumigatus
This chart visualizes the efficacy of various antifungal agents in treating Aspergillus fumigatus. Blue bars represent overall response rates, while green bars indicate reductions in mortality. Combination therapy shows superior outcomes across both metrics based on BMC Infectious Diseases data.
First-line antifungal therapy:
- Voriconazole – Often regarded as the gold standard, voriconazole is effective in approximately 60–70% of invasive aspergillosis cases. Administered orally or intravenously, the treatment typically begins with a loading dose followed by maintenance dosing. Side effects may include visual disturbances and liver enzyme elevations. The average monthly cost in the U.S. ranges from $2,000 to $4,000, although generic options have made it more accessible. It remains the most recommended option by infectious disease specialists.
- Isavuconazole – A newer-generation triazole antifungal, isavuconazole offers similar efficacy to voriconazole but with fewer side effects and no requirement for cyclodextrin (a solubilizing agent with renal toxicity risks). It’s especially preferred in patients with kidney dysfunction. A full treatment course can cost $3,500–$6,000. Isavuconazole is increasingly used in both hospital and outpatient settings, and clinical guidelines increasingly endorse it as an alternative first-line option.
- Liposomal Amphotericin B – This formulation minimizes the nephrotoxicity traditionally associated with amphotericin B. It is reserved for patients who either do not respond to azoles or have confirmed azole-resistant A. fumigatus. It’s administered intravenously and may cost upwards of $1,000 per day in inpatient care. Despite the cost, it is highly valued in critical care units for its broad-spectrum potency.
New Innovations:
- Brexafemme (ibrexafungerp): While FDA-approved for treating vaginal yeast infections, brexafemme is being trialed for invasive fungal infections. It works by inhibiting glucan synthase, disrupting the fungal cell wall. Currently not in wide clinical use for aspergillosis, but expected to enter advanced trial phases in 2025–2026. Cost and dosing data for off-label use remain limited.
- Molecular resistance profiling: Rapid genetic testing can now identify azole-resistant strains within hours. These assays are becoming routine in major U.S. transplant centers and reduce the risk of treatment delays. Costs range from $400–$700 per test, and while not universally covered, many academic centers provide access during hospitalization.
- Nebulized antifungals: Delivering drugs like amphotericin B directly to the lungs via nebulization reduces systemic toxicity. Used adjunctively, especially in ICU or post-transplant settings. Portable nebulizer kits cost around $300–$600, while the drug itself varies in cost depending on formulation. This method is gaining popularity among pulmonologists for targeted therapy.
Update 2025:
- siRNA Therapy: In 2025, clinical research showcased the potential of small interfering RNA (siRNA) treatments that silence essential fungal genes. Delivered using anionic liposomes combined with Amphotericin B, this method showed strong efficacy in vitro and in early clinical models. This treatment is still experimental and not yet commercially available but could revolutionize therapy within the next 3–5 years.
- Mandimycin: A newly identified antifungal, mandimycin, is currently in preclinical testing. It operates by disrupting fungal protein synthesis and has demonstrated high potency against resistant strains. Though not yet approved, early-phase studies indicate it could complement or replace existing therapies when azoles fail.
Overall, treatment success depends on early diagnosis, resistance profiling, and timely initiation of antifungal therapy. Combination therapy and innovations like nebulization or RNA-based treatments are enhancing outcomes, especially in severe or resistant cases. As always, physician guidance is critical, and patients should follow up regularly to monitor response and side effects.
Real-World Case Highlight
In California, a 45-year-old woman recovering from a stem cell transplant developed invasive pulmonary aspergillosis. Rapid PCR testing and galactomannan assay led to early detection. She was treated successfully with voriconazole and adjunct nebulized amphotericin B.
Preventive Measures
Preventing exposure to Aspergillus fumigatus, especially for immunocompromised individuals, involves multiple coordinated strategies. Here’s a closer look at how each measure works, how effective it is, and how it can be implemented in everyday life:
- Avoid exposure to compost, moldy hay, or construction dust: These environments are high-risk zones due to heavy fungal spore concentration. For high-risk individuals, simple actions—like wearing a NIOSH-approved N95 mask while gardening or avoiding renovation areas—can significantly reduce exposure. This preventive step is estimated to cut down inhalation of Aspergillus spores by over 80% in controlled environments.
- Use HEPA filters in hospitals and at home for high-risk individuals: High-Efficiency Particulate Air (HEPA) filters can trap particles as small as 0.3 microns, effectively removing fungal spores from the air. In clinical settings, these are used in oncology wards and transplant units. At home, portable HEPA air purifiers or integrated HVAC filters can create safer breathing environments. Replace filters every 2–3 months and monitor indoor humidity (ideally below 50%).
- Prophylactic antifungal treatment for transplant and oncology patients: Prophylaxis with medications like posaconazole or isavuconazole is now standard in many transplant centers. This preventive approach can reduce the risk of invasive aspergillosis by up to 75%, particularly in patients undergoing hematopoietic stem cell transplant or chemotherapy. Physicians determine eligibility based on immune status and infection risk scores.
- Regular screening for at-risk populations: Screening methods like galactomannan assays and chest CT scans are used proactively in high-risk hospitalized patients, such as those with prolonged neutropenia. Early detection through these means leads to better outcomes and avoids the need for aggressive treatment later. For outpatients, periodic check-ups and prompt evaluation of new respiratory symptoms are key.
What to Watch for: Individuals with chronic lung disease, recent hospitalization, or immune suppression should stay alert for subtle signs such as a persistent dry cough, mild fever, or breathlessness—especially after environmental exposure. Timely consultation with a specialist can prevent a minor infection from becoming invasive.
Implementing these strategies in combination rather than isolation significantly enhances their effectiveness. As always, awareness and consistency are the cornerstones of prevention.
Editorial Advice
Reyus Mammadli, healthcare advisor, recommends that clinicians and healthcare systems ramp up surveillance for azole-resistant Aspergillus fumigatus. “We’re seeing more resistance, and it’s crucial to test before treating. Guesswork leads to poor outcomes,” he says.
Also, don’t ignore subtle symptoms. Persistent cough, unexplained fatigue, or fever in an immunocompromised patient warrants a fungal workup. As fungal threats quietly evolve, our diagnostic and therapeutic tools must evolve even faster.
References
- Nature Microbiology (2022) – “Environmental azole resistance in Aspergillus fumigatus.” Estimates resistance in ~20% of environmental strains.
- European Centre for Disease Prevention and Control (ECDC) (2025) – Risk assessment on environmental fungal threats and climate-driven expansion.
- CDC – Aspergillosis – Overview of risk factors, symptoms, and forms of disease.
- PubMed: Panther Fusion System in Fungal Diagnostics (2024) – Study on multiplex PCR-based detection.
- Science Advances (2024) – Use of Tip-Enhanced Raman Spectroscopy in fungal diagnostics.
- Clinical Infectious Diseases Journal (2023) – Efficacy and side effects of voriconazole and isavuconazole.
- Journal of Antimicrobial Chemotherapy (2025) – RNA interference therapy in fungal infections.
- NIH Clinical Trials Database – Mandimycin and Brexafemme in pre-clinical/clinical testing for invasive fungal infections.
- Mayo Clinic – HEPA filter effectiveness and environmental exposure guidelines.
- Journal of Fungi (2023) – Cost-effectiveness and hospital protocols in antifungal prophylaxis.
About the Author
Reyus Mammadli is the author of this health blog since 2008. With a background in medical and biotechnical devices, he has over 15 years of experience working with medical literature and expert guidelines from WHO, CDC, Mayo Clinic, and others. His goal is to present clear, accurate health information for everyday readers — not as a substitute for medical advice.