Introduction
Methotrexate is a widely used medication known for its effectiveness in treating a variety of conditions, including rheumatoid arthritis, psoriasis, and certain types of cancer. However, its use during pregnancy has raised significant concerns due to its potential risks to both the mother and the developing fetus. This article aims to provide a comprehensive overview of methotrexate, focusing on its safety during pregnancy, potential risks, and alternative treatment options.
Methotrexate: An Overview
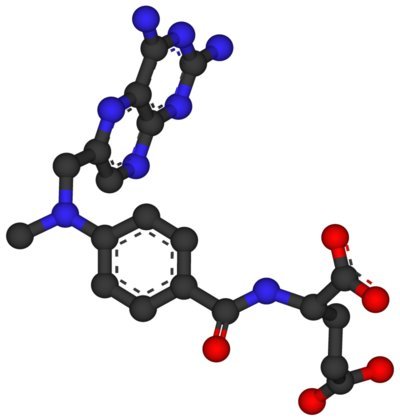
Methotrexate is an antimetabolite and antifolate drug that inhibits the enzyme dihydrofolate reductase, thereby interfering with DNA synthesis and cell replication. It is commonly prescribed in low doses for chronic conditions such as rheumatoid arthritis and psoriasis, and in higher doses for certain cancers, including leukemia and breast cancer. Due to its mechanism of action, methotrexate can have teratogenic effects, which means it can cause developmental abnormalities in the fetus.
Methotrexate and Pregnancy
Methotrexate is classified as a Category X drug by the U.S. Food and Drug Administration (FDA), indicating that it is contraindicated during pregnancy. This classification is based on evidence from both animal studies and human case reports that demonstrate significant risks of fetal harm when the drug is administered during pregnancy.
Risks to the Fetus
The use of methotrexate during pregnancy, particularly during the first trimester, has been associated with a range of congenital abnormalities, collectively known as “fetal methotrexate syndrome.” These abnormalities can include craniofacial deformities, limb defects, and growth retardation. The risk of miscarriage is also significantly increased when methotrexate is used during pregnancy.
Risks to the Mother
For pregnant women, the use of methotrexate can lead to serious complications, including hematological disorders and liver toxicity. Additionally, the drug’s immunosuppressive effects can increase the risk of infections, which can further complicate pregnancy outcomes.
Alternatives to Methotrexate During Pregnancy
Given the risks associated with methotrexate, it is crucial for healthcare providers to explore alternative treatment options for pregnant women or those planning to become pregnant. For conditions such as rheumatoid arthritis, safer alternatives may include medications like sulfasalazine, hydroxychloroquine, or biologic agents that have a better safety profile during pregnancy. For cancer treatment, the timing of therapy may need to be adjusted, or alternative chemotherapeutic agents may be considered.
Preconception Counseling and Family Planning
For women of childbearing age who are on methotrexate, preconception counseling is essential. It is recommended that methotrexate be discontinued at least three months before attempting to conceive to minimize the risk of fetal exposure. Additionally, effective contraception should be used during methotrexate therapy to prevent unintended pregnancies.
Conclusion
Methotrexate is a powerful medication with proven efficacy in treating a range of conditions. However, its use during pregnancy carries significant risks to both the mother and the developing fetus. Healthcare providers must carefully weigh the benefits and risks of methotrexate in pregnant patients and consider alternative treatment options whenever possible. For women of childbearing potential, preconception counseling and family planning are critical to ensure the safety of both the mother and the future child.
References
- U.S. Food and Drug Administration. Methotrexate.
- American College of Rheumatology. Guidelines for the Management of Rheumatoid Arthritis.
- World Health Organization. Safe medication use during pregnancy.
Disclaimer: This article is intended for informational purposes only and does not constitute medical advice. Please consult a healthcare provider for personalized recommendations.
About the Author
Reyus Mammadli is the author of this health blog since 2008. With a background in medical and biotechnical devices, he has over 15 years of experience working with medical literature and expert guidelines from WHO, CDC, Mayo Clinic, and others. His goal is to present clear, accurate health information for everyday readers — not as a substitute for medical advice.